Daniel Hudson, UVM Extension Agronomist
- Minerals containing aluminum are natural and abundant in the soil.
- Acidic soils increase ‘free aluminum’ levels, which bind soluble (plant-available) phosphate, can cause plant toxicity, and ultimately can significantly reduce crop yield and quality.
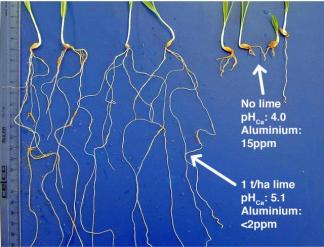
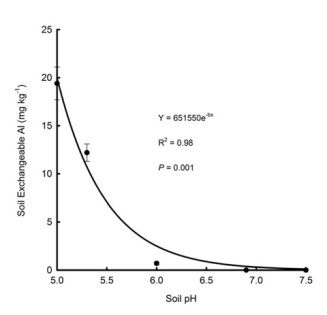
- Lime does not instantaneously correct soil pH and aluminum-related problems. While visible improvements can be observed even in the same season, it can take years for lime to fully mitigate soil acidity and reduce levels of free aluminum.
- Soil testing every three years and following soil test recommendations will reduce the likelihood of developing aluminum-involved crop production problems.
——
Things that are invisible are not necessarily unimportant: oxygen, carbon monoxide, and gravity, to name a few. This is as true in the soil as it is above ground. While we tend to think of soil as ‘sand, silt, and clay’,
there are many tremendously important things in it that are invisible or barely visible to the unaided eye. This is true for much of the soil ecosystem: bacteria, viruses, massive webs of microscopic fungi, frightful soil insects, hideous mites, sprawling plant roots, decaying plant material, all sorts of [naturally occurring] chemicals. Soil organism populations oscillate wildly, depending on environmental conditions, complex chemical reactions are occurring everywhere – it’s a war where chemical and biological weapons are the norm, and yet somehow there is order, beauty, and a little predictability to it! And yet, most of it is invisible.
Let’s zoom in on one economically important yet underappreciated element found in soil: aluminum. Unless someone has previously drawn your attention to soil aluminum, you may not have thought much about it before. It sounds terribly uninteresting, doesn’t it?! Read on only if you think crop yield, quality, and farm profitability are interesting, because aluminum is involved in all of them.
How did aluminum get in my soil?
Several years ago I had a conversation with an individual who was surprised and not a little disturbed that aluminum had somehow made its way into their soils! It was probably a comfort to them to learn that aluminum is natural and abundant in the earth’s crust, and is not just the result of littering, government conspiracies, industrial pollution, or the Russian government. In fact, aluminum accounts for a solid 8% of the weight of the earth’s crust, making it the third most abundant element on earth, coming in behind oxygen and silicon.
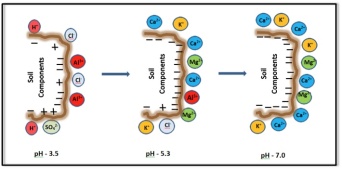
Maybe someone will find an exception someday, but as far as I know, aluminum is not an essential nutrient for any living organism. For the most part, aluminum is a component of many stable soil minerals that are perfectly safe to handle and for plants to grow in. Soil aluminum becomes problematic in many cropping systems when the soils are acidic (i.e., low pH). Under acidic soil conditions, aluminum increasingly shifts from the insoluble mineral phase to soluble phases that are often referred to as ‘free aluminum’ and denoted ‘Al3+’. Free aluminum presents several costly agronomic problems.
If free aluminum is really a problem, why have I never heard of it?

Closer corn and soy have pH –> Al-induced agronomic problems
Free aluminum is present at crop-toxic levels in 1.7 billion acres in the tropics alone, and can be implicated, at least in a secondary way, in widespread poverty, malnutrition, and starvation in those regions. In the Northeast U.S. aluminum-involved soil fertility problems can (I estimate) result in yield losses of more than 30% when not managed appropriately. Further losses arise from reduced forage quality. Between lost crop yield and reduced forage quality, the total economic impact of free aluminum in the soil can easily surpass $200/ac/year in dairy cropping systems. It is safe to say that free aluminum has caused some farms/fields to perform miserably, and even to fail.
The main reason we do not hear more about free aluminum in the soil in the U.S. is because we tend to talk more about soil pH than we do aluminum. Soil pH has a powerful influence on soil chemistry. Increasing acidity causes free aluminum to be much more abundant, and this causes many ‘downstream’ agronomic effects.
For the most part, the focus on pH rather than aluminum is appropriate because 1) we cannot physically remove aluminum from the soil; 2) aluminum-related problems are strongly correlated with soil pH; 3) the aluminum-related problems can be generally, over time, be mitigated by adjusting the pH; and 4) pH affects more than just aluminum ability: while all are essential plant nutrients, iron, manganese, and calcium can all cause nutrient-related problems at certain ranges of soil pH.
At a moderately low pH (4.5-6.2), the primary problems that free aluminum presents to growing crops are:
- Binding (immobilizing) soluble phosphate, making it unavailable for crop
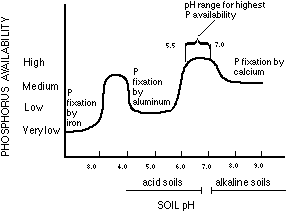
from: http://www.extension.umn.edu/agriculture/nutrient-management/phosphorus/the-nature-of-phosphorus/
uptake. As much fuss as we make about phosphorus management, in Vermont, MANY acres of Vermont crop land are severely deficient in phosphorus. To a great extent, this problem is induced by soil acidity. The free aluminum released as the result of soil acidity gladly and strongly binds soluble phosphate, making it unavailable for crop uptake.
- Displacing other positively charged plant nutrients that, unlike aluminum, ARE essential. ‘Cation exchange capacity ‘ (CEC on your soil test report) is an indication of the ability of a particular soil to store positively charged atoms (ions) such as calcium (Ca2+), potassium (K+), and magnesium (Mg2+). Think of CEC it as the ‘pantry’ of the soil. Typical CEC levels in agricultural soils in Vermont soils range from 4 to 25
meq/100g. Clay and soil organic matter increase CEC, and are essentially the shelves in the pantry. Under acidic conditions, free aluminum resolutely occupies more of the CEC positions and displaces a variable proportion of those other nutrients that would otherwise be in those positions.
Good news and bad news
Given that free aluminum is and always has been 1) abundant; 2) non-essential; 3) biologically problematic, it is not surprising that many organisms have ways to manage its presence in their environment. One of the best understood defenses plant roots have is the secretion of organic acids (such as citric and malic acids) that ‘chelate’ (i.e. bind) free aluminum. That is the good news.
The bad news is that 1) all defenses have limits; and 2) certain plant species have better defenses against aluminum toxicity than others. Below a pH of 4.5, the abundance of free aluminum is so overwhelming to many plant species that the normal plant defense mechanisms are often not sufficient. In these situations, direct and indirect toxicity to plant roots and systems commonly include:
- Stunted primary root growth by inhibiting cell division and elongation
- Inhibition of lateral root formation.
- Reduced root diameter, and increased root brittleness.
- Reducing root hair development.
- Damaged structure and disrupted function of cell membranes.
- More random root branching patterns.
- Disrupted signaling/communication pathways within and among plant cells.
- Interference with the uptake and metabolism of essential nutrients (at the molecular level, not just due to poor root development).
- Increased susceptibility to secondary (opportunistic) diseases.
- Not surprisingly, reduced water and nutrient uptake, and ultimately reduced crop yield.
My soil pH is 5.6, which is high enough that free aluminum toxicity should not be a problem!
There are two things to keep in mind before concluding that your moderate acidity is not an agronomic and economic problem on your farm. First, under such conditions, phosphorus availability is being negatively impacted by free aluminum. While high phosphorus levels are a problem in some fields in Vermont, many of the soil test reports I see from Eastern Vermont are on the low side. Some are extremely low. Because there is a very strong relationship between available soil phosphate and crop yield, farmers should be alarmed when their soil test reports indicate that extractable phosphate is below the optimal level. Secondly, if the AVERAGE soil pH is 5.6, you probably have many zones (large and small) where the pH is much lower and where aluminum-related agronomic problems are more obvious. Some of those zones will have toxic effects on the roots – rendering the nutrients and water in those zones less available for uptake by the affected plant. The picture to the right shows a field that  was, on average above 6.2, but the corner pictured ranged from pH 4.0 (the wheat that is almost dead) to 4.4 (the healthier looking wheat).
was, on average above 6.2, but the corner pictured ranged from pH 4.0 (the wheat that is almost dead) to 4.4 (the healthier looking wheat).
For many fields in the Northeast U.S., mitigating and preventing the development of severe soil acidity should be a top priority. Unless lime is incorporated into the soil, it can take years for the lime to move into and neutralize the pH in the top several inches of soil. Thus, if you have a severe soil acidity situation and the soil conditions are well-suited for it, consider incorporating the lime to hasten the effect.
Reference material:
Delhaize, E., and P. Ryan. Aluminum toxicity and tolerance in plants. Plant Physiol. (1995) 107:315-321. Online.
Dragana Krstic, Ivica Djalovic, Dragoslav Nikezic and Dragana Bjelic (2012). Aluminium in Acid Soils: Chemistry, Toxicity and Impact on Maize Plants. Food Production – Approaches, Challenges and Tasks, Prof. Anna Aladjadjiyan (Ed.), ISBN: 978-953-307-887-8, InTech. Available online.
Harter, R. D., Acid Soils of the Tropics. ECHO Technical Note. 2007. Online.
University of Hawaii at Manoa. Highly weathered tropical soils. Online
University of Hawaii at Manoa. Soil Mineralogy. Online
Panda, S.K., Baluska, F., and H. Matsumoto. Aluminum stress signaling in plants. in Plant Signal Behavior. July 2009; 4(7): 592-597.
Scheffer-Basso, S., B. Prior. Aluminum toxicity in roots of legume seedlings assessed by topological analysis. Acta Sci., Agron. vol.37 no.1. Jan./Mar. 2015. Online.
Schulte, E.E., and K.A. Kelling. A2520 – Soil and applied phosphorus. Understanding Plant Nutrients. 1996. University of Wisconsin Extension. Online.
Victoria Agriculture. Acid Soils. April 2005. Note Number: AG1182. Online.